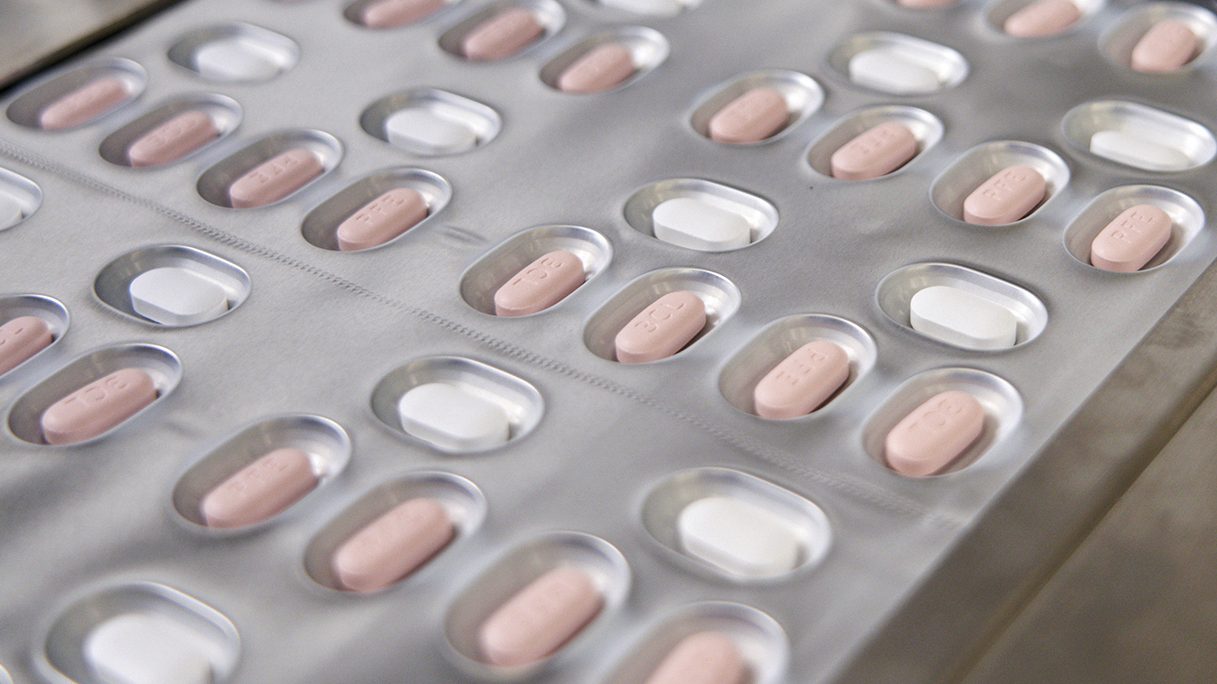
Two newly-authorized antiviral pills for treating COVID-19 will be "in the coming weeks" in Illinois, health officials announced Thursday.
The pills Paxlovid and Molnupiravir will be available by prescription only "for those with mild-to-moderate COVID-19 who are at high risk for becoming severely ill, including hospitalization or death," according to the Illinois Department of Public Health.
While IDPH did not give a provide a date for when the pills will be available, the department said it is working with pharmacies statewide, including Walgreens and Walmart to carry the antiviral medications. A complete list of locations is expected to be released in the coming weeks.
"While these antivirals are free from the federal government, they are in limited supply," IDPH said in a news release. "Illinois will receive a renewed allocation of antivirals every two weeks.
IDPH noted that the pills should be taken "as soon as possible after being diagnosed and within five days of the beginning of symptoms."
U.S. regulators authorized Pfizer's pill, Paxlovid, and Merck’s molnupiravir last month. In high-risk patients, both were shown to reduce the chances of hospitalization or death from COVID-19, although Pfizer's was much more effective.
IDPH reported Paxlovid is expected to reduce the risk of hospitalizations by 89% and Molnupiravir by about 30%.
The antiviral pills aren’t for everyone who receives a positive test results.
They are intended for those with mild or moderate COVID-19 who are more likely to become seriously ill. That includes older people and those with other health conditions like heart disease, cancer or diabetes that make them more vulnerable.
Feeling out of the loop? We'll catch you up on the Chicago news you need to know. Sign up for the weekly Chicago Catch-Up newsletter here.
Both pills were OK'd for adults while Paxlovid is authorized for children ages 12 and older.
Merck’s molnupiravir is not authorized for children because it might interfere with bone growth. It also isn't recommended for pregnant women because of the potential for birth defects.
Pfizer's pill isn't recommended for patients with severe kidney or liver problems. It also may not be the best option for some because it may interact with other prescriptions a patient is taking.
The antiviral pills aren't authorized for people hospitalized with COVID-19.
Molnupiravir is meant for use when other treatment options are not available.
In addition to the pills, people can still receive monoclonal antibody treatment, which can "help prevent COVID-19 from progressing to a point where a person needs to be hospitalized." Prescriptions are required for both medications.