What if light therapy could stop the spread of coronavirus by eliminating the viral load in your nose? One Canadian company with offices in Chicago says it has the technology to make that happen. NBC 5’s Lauren Petty reports.
What if light therapy could stop the spread of the coronavirus by eliminating the viral load in your nose? One Canadian company with offices in Chicago says it has the technology to make that happen.
“This is a very simple intervention. It takes a couple minutes. It's inexpensive. It's portable,” said Dr. Nicolas Loebel, Chief Technology Officer at Ondine Biomedical, the company that developed the Steriwave Nasal Decolonization Technology. “In our case, we don't see any adverse effects, (or) any significant adverse effects, over a decade in 60,000 patients."
Approved in Canada for use before surgery to help prevent infections including MRSA, Ondine Biomedical says photodisinfection therapy (PDT) can also fight this pandemic.
“We know it works against this particular virus. And so now the real question is, when we remove a virus from the areas we can access, what's the outcome? Can we buy patients time? Can we buy healthcare workers time?” Dr. Loebel said.
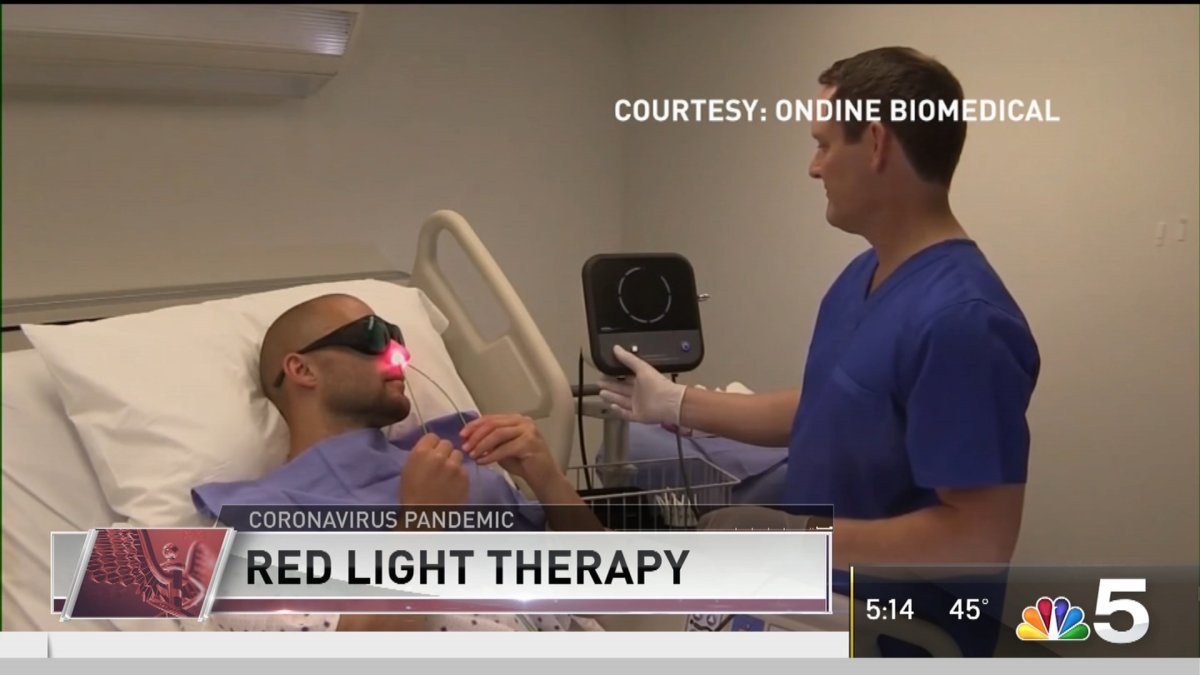
Studies have shown the coronavirus tends to colonize in the nose, so the process involves swabbing the nose with a blue-colored chemical compound. Then a technician inserts fiber optic probes into the nose and turns on a red light, the kind of light found at the safe end of the spectrum.
“When you shine that light in the presence of the photo sensitizer that's on the virus, It basically blows the virus up. It destroys it immediately,” said Dr. Merrill Biel, an otolaryngologist and professor at the University of Minnesota.
Dr. Biel has been studying photo dynamic therapy since 1987 and says the science is there to warrant further study during the pandemic.
Local
“It's really establishing the clinical trial, which is very doable. It's a very doable trial, a painless short treatment,” said Dr. Biel.
Ondine Biomedical says studies are underway in Canada and they are in discussions with the United States Food and Drug Administration to launch trials in the U.S.
“We've discussed emergency use authorization, expanded access, also called compassionate use, and even just a simple straight clinical study, as though we were not in a COVID-19 pandemic,” said Dr. Loebel.
The company's goal would be to disinfect frontline workers first, possibly after every shift.
“We believe that the therapy will provide substantial efficacy with so low toxicity, that it can be repeated as often as once per day, once per nursing shift,” Dr. Loebel said.
It would not replace the need for masks, face shields and other PPE, but work in conjunction with it, if the technology gets FDA approval, according to the physicians.