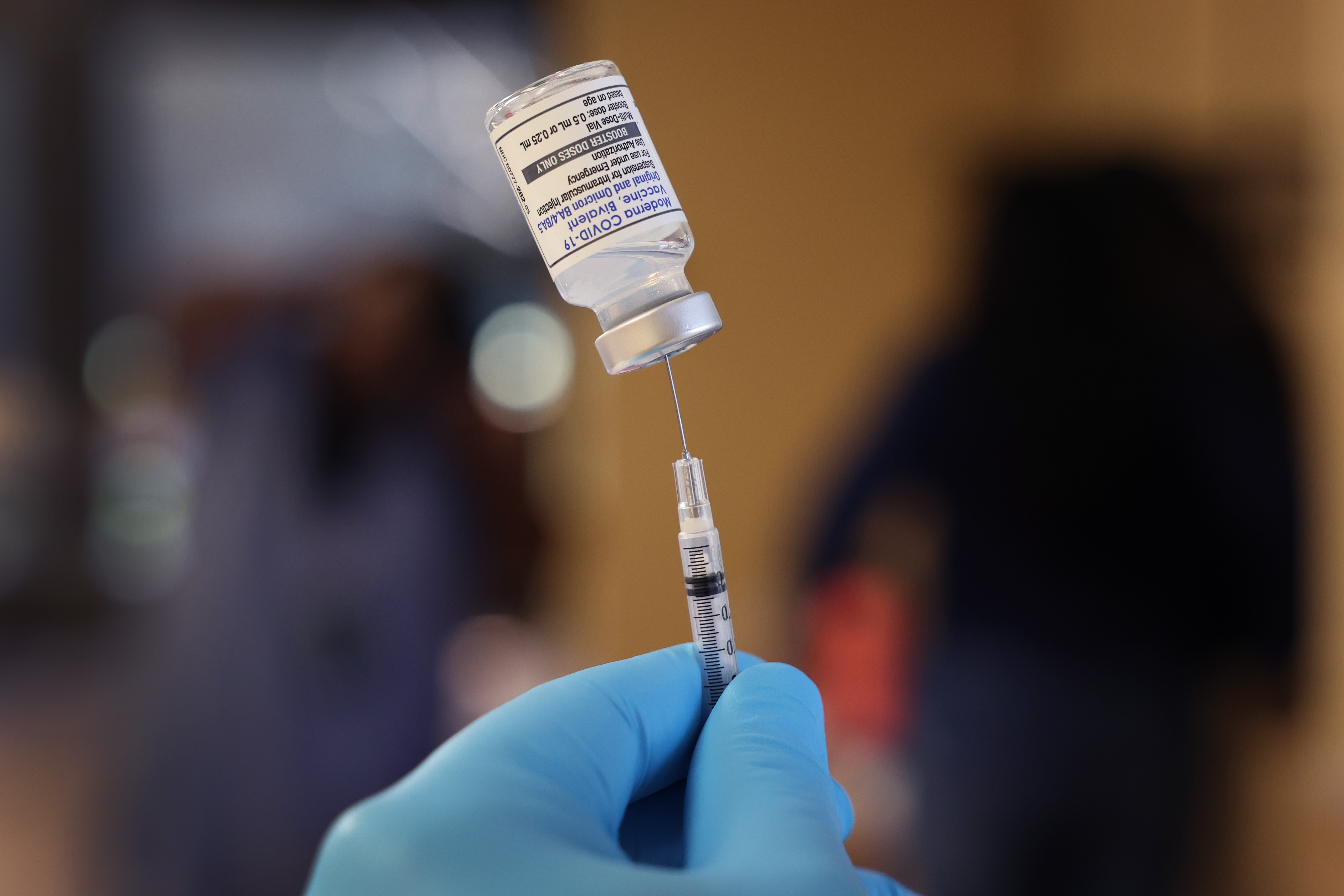
With a new COVID-19 vaccine targeting recent variants surfacing this month, many are wondering what's different about these vaccines and what side effects could be experienced after getting boosted.
The new vaccines are known as "bivalent," meaning the new doses contain half of the original vaccine recipe with half of the vaccine providing protection against the new omicron variants, BA.4 and BA.5.
Vaccines continued to show effective protection, even regarding different strains, and now, experts hope the newest shots can go even further and provide additional protection.
With just around 1.5 percent of those eligible to receive an updated booster have gotten one, there is limited data thus far on potential side effects of the booster shots.
Feeling out of the loop? We'll catch you up on the Chicago news you need to know. Sign up for the weekly Chicago Catch-Up newsletter here.
However, health experts suggest side effects will likely be similar to previous doses, with arm pain, fatigue, headache and nausea being reported as side effects.
With such a small amount of Americans having received the dose so far, some health experts are expressing concern as cases are expected to rise as weather gets colder in much of the country.
“I would expect a much higher proportion of Americans to have gotten the booster by this point,” Dr. Scott Roberts with Yale Medicine told NBC News.
As the fall season begins and cooler weather settles in, health officials are urging boosters in an effort to prevent another possible surge.
“Colder weather is coming and residents are starting to move indoors, which is traditionally when we see respiratory virus rates rise. Please don’t wait to get vaccinated this year. Do it now to protect yourself, your family, and our whole city,” Dr. Allison Arwady, commissioner of the Chicago Department of Public Health, said in a news release Friday.
If you're planning to get the latest shot or are unsure, here's what you need to know, including possible side effects, where shots are being offered and who should receive them.
What Are the Possible Side Effects?
Side effects caused by the boosters may not be that different from your last dose.
"We just don't have any data on this [yet], essentially giving two vaccines in one shot — but biologically, I just wouldn't expect the side effects, severity or the safety profile of the shots to be different from the current mRNA vaccines and boosters," Dr. Paul Offit, director of the Vaccine Education Center at Children's Hospital of Philadelphia and member of an independent advisory group to the U.S. Food and Drug Administration, told CNBC's Make It.
The Food and Drug Administration states that those who receive the bivalent vaccine "may experience side effects commonly reported by individuals who receive authorized or approved monovalent mRNA COVID-19 vaccines."
Among the side effects study participants reported were:
- pain, redness or swelling where the shot was administered
- fatigue
- headache
- muscle pain
- join pain
- chills
- swelling of the lymph nodes in the arm where the shot was given
- nausea or vomiting
- fever
The side effects were similar for both Moderna and Pfizer's vaccines and largely mirror expected side effects for earlier doses.
The CDC stated that side effects with the third shot were also "similar to that of the two-dose series."
The most common symptoms then included fatigue and pain at the injection site, but "most symptoms were mild to moderate."
As with previous doses of the vaccine, the CDC notes that, "serious side effects are rare, but may occur."
Who is Eligible?
According to the Centers for Disease Control and Prevention, only those who have completed a full COVID vaccine series -- which consists of either two Moderna or Pfizer shots, or one Johnson & Johnson shot -- are eligible. Additionally, the shots have certain age restrictions, which are listed below:
- Individuals 18 and older are eligible to receive either Pfizer’s or Moderna’s updated COVID booster shot
- Only Pfizer booster doses can be administered to those aged 12 through 17
- While those younger than 18 years old are eligible for the new COVID booster, they aren't eligible for the Moderna dose
Where Can I Go to Get the Updated Booster?
Walgreens and CVS are among those offering updated booster shots, along with several other retail chains. Walgreens encourages anyone eligible to schedule an appointment via the Walgreens app, by calling 1-800-WALGREENS or going online. Walk-ins are permitted, though appointments are preferred.
CVS, too, encourages those interested to schedule appointments online, according to a news release about the rollout earlier this month. At the time, CVS stated initial supply of the updated boosters was limited.
Will Children Be Eligible Soon?
Moderna announced Friday it asked the FDA to authorize its booster for children, explaining it filed two separate authorization requests - one for those 12 to 17 years old and another for kids age 6 to 11.
This week, the CDC said it anticipates recommending updated boosters for kids between early and mid-October. Pfizer has informed a CDC advisory committee that it plans to ask the FDA to authorize boosters for children age 5 to 11 in early October.
Can You Mix and Match Your Booster Shot?
While the Centers for Disease Control and Prevention does not recommend mixing products for your primary series doses, boosters can be mixed.
Here's the CDC guidance on mixing and matching for boosters, based on which shots you have already received.
- People ages 18 years and older may get a different product for a booster than they got for their primary series, as long as it’s Pfizer-BioNTech or Moderna.
- Teens ages 12-17 years may get a different product for a booster than they got for their primary series, as long as it’s Pfizer-BioNTech.
- Children ages 5 through 11 years who got a Pfizer-BioNTech primary series must also get Pfizer-BioNTech for a booster.
- People ages 12 years and older may only get the updated (bivalent) mRNA (Pfizer-BioNTech or Moderna) booster. They can no longer get an original (monovalent) mRNA booster.
- Novavax is not authorized for use as a booster dose at this time.