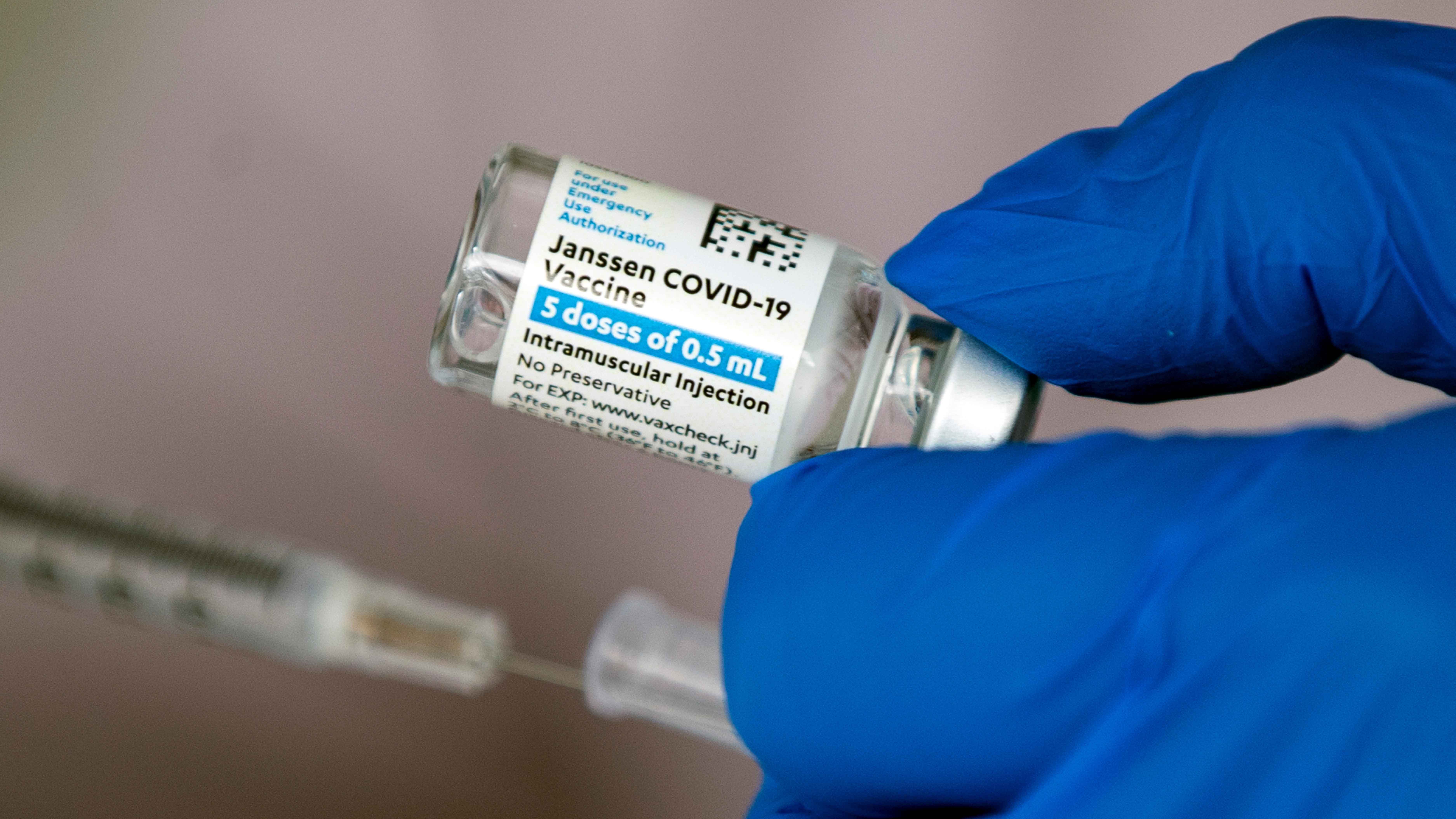
What to Know
- Illinois will pause use of the Johnson & Johnson vaccine "out of an abundance of caution"
- Health officials say a majority of Illinois' vaccine doses are from Moderna and Pfizer's vaccines
- U.S. regulators recommended a “pause” in administration of the single-dose Johnson & Johnson COVID-19 vaccine to investigate reports of potentially dangerous blood clots
Illinois will pause use of the Johnson & Johnson vaccine "out of an abundance of caution" following a recommendation from the U.S. Centers for Disease Control and Prevention and the U.S. Food and Drug Administration, the state's health department said Tuesday.
"IDPH has notified all Illinois COVID-19 providers throughout the state to discontinue use of the J&J vaccine at this time," the Illinois Department of Public Health said in a statement. "In order to keep appointments, IDPH is strongly advising providers to use Pfizer-BioNTech and Moderna vaccines."
Health officials say a majority of Illinois' vaccine doses are from Moderna and Pfizer's vaccines. Of the expected 483,720 doses the state is set to receive next week, 5,800 were set to be Johnson & Johnson.
Feeling out of the loop? We'll catch you up on the Chicago news you need to know. Sign up for the weekly Chicago Catch-Up newsletter here.
This week, the state had received 17,000 doses of the Johnson & Johnson vaccine, officials said.
"IDPH will continue to update the public as additional information becomes available," the department's statement read.
The state's decision comes as the U.S. regulators recommended a “pause” in administration of the single-dose Johnson & Johnson COVID-19 vaccine to investigate reports of potentially dangerous blood clots.
In a joint statement Tuesday, the CDC and FDA said they were investigating unusual clots in six women that occurred 6 to 13 days after vaccination. The clots occurred in veins that drain blood from the brain and occurred together with low platelets. All six cases were in women between the ages of 18 and 48.
Officials are recommending that people who were given the J&J vaccine who are experiencing severe headache, abdominal pain, leg pain, or shortness of breath within three weeks after receiving the shot contact their health care provider.
The reports appear similar to a rare, unusual type of clotting disorder that European authorities say is possibly linked to another COVID-19 vaccine not yet cleared in the U.S., from AstraZeneca.
More than 6.8 million doses of the J&J vaccine have been administered in the U.S., the vast majority with no or mild side effects.
U.S. federal distribution channels, including mass vaccination sites, will pause the use of the J&J shot, and states and other providers are expected to follow. The other two authorized vaccines, from Moderna and Pfizer, make up the vast share of COVID-19 shots administered in the U.S. and are not affected by the pause.
Cook County is also pausing use of the Johnson & Johnson COVID-19 vaccine, county health officials said Tuesday.
"Following guidance released this morning from the US Food and Drug Administration and the US Centers for Disease Control and Prevention, Cook County Health will pause the use of the Johnson & Johnson vaccine until the FDA and CDC complete their review," a spokeswoman for Cook County Health said in a statement Tuesday morning.
Individuals with appointments for the Johnson & Johnson vaccine this week will instead receive the first dose of either Moderna or Pfizer's vaccine, depending on the vaccination site, the county said. Anyone who has a scheduled appointment but does not want the Moderna or the Pfizer vaccine should call 833-308-1988 to cancel or reschedule their appointment, officials said.
An advisory committee is scheduled to meet Wednesday to review the reactions and consider how to proceed.
Officials say they also want to educate vaccine providers and health professionals about the “unique treatment” required for this type of clot.