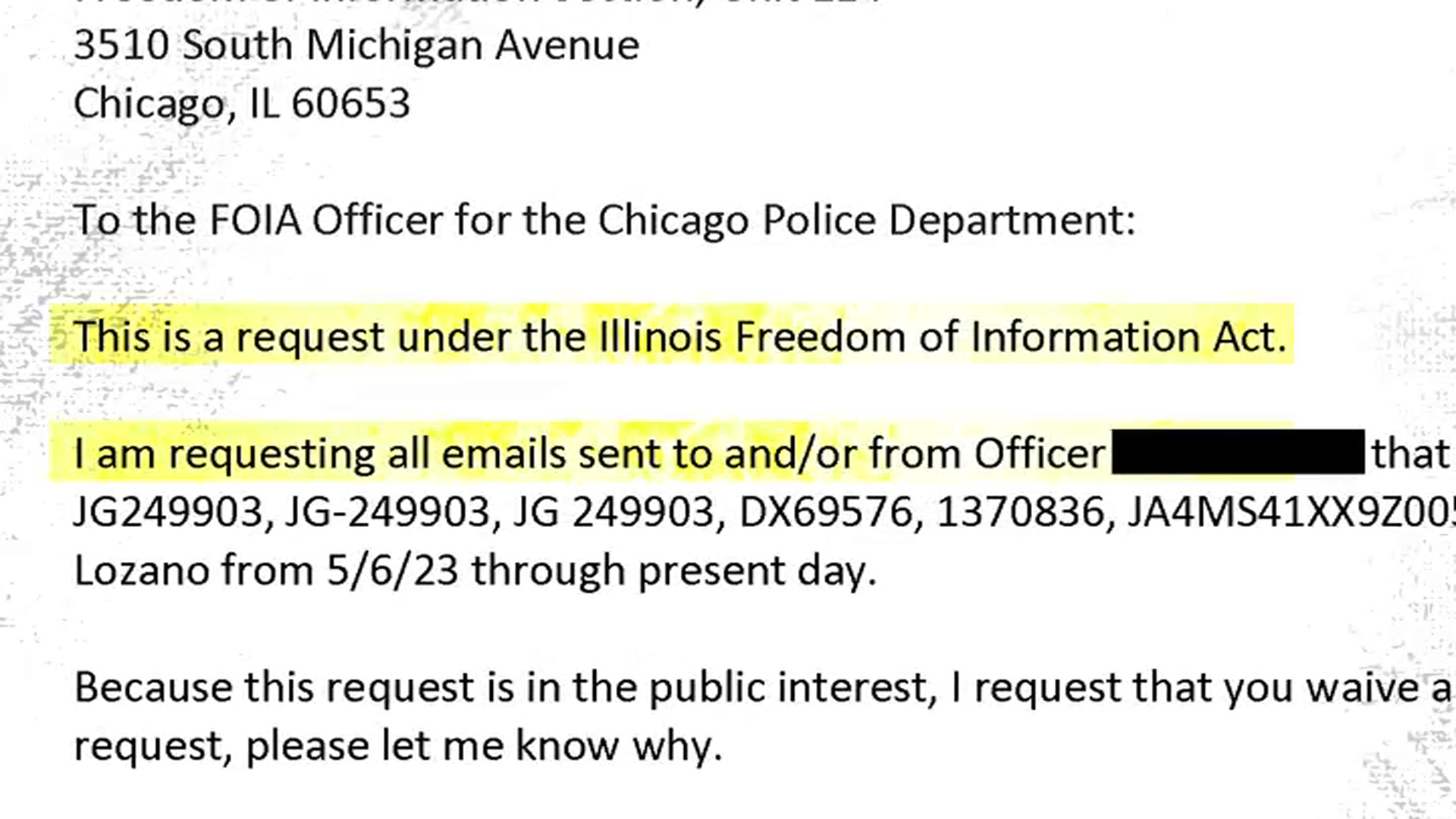
As the Food and Drug Administration gives Pfizer's child-size COVID vaccine emergency use authorization Friday, children as young as 5 could soon be eligible for coronavirus vaccines nationwide.
However, one more step must be taken.
Full-strength Pfizer shots already are recommended for anyone 12 or older, but pediatricians and many parents are anxiously awaiting protection for younger children to stem infections from the extra-contagious delta variant and help keep kids in school.
Friday's vote was the next step in a review process that will still require the Centers for Disease Control and Prevention to approve the vaccine.
Feeling out of the loop? We'll catch you up on the Chicago news you need to know. Sign up for the weekly Chicago Catch-Up newsletter here.
The CDC scheduled an advisory committee meeting to review the doses next week and is expected to authorize them for public distribution immediately thereafter.
Should the CDC approve, children could begin vaccinations in early November -- with the first kids in line fully protected by Christmas.
Illinois Gov. J.B. Pritzker said final approval for vaccines in this age group could come in the middle of next week, clearing the way for the state to begin vaccinations.
Local
"In just a few days time, millions of parents all across the United States should be able to breathe a sigh of relief that they've been holding in for over 18 months now," Pritzker said.
Dr. Nimmi Rajagopal, the associate chair of the Department of Family and Community Medicine for Cook County Health, said the system is "absolutely preparing" for the vaccine to be approved for children.
"We have plans for how we will communicate the official approval with our patients as well as anyone that registered for or had a vaccine of one of our sites," Rajagopal said. "So our system is preparing and ready to go when we get the vaccine and they get the approval."
Federal health regulators said that child-size doses of Pfizer’s COVID-19 vaccine appear highly effective at preventing symptomatic infections in elementary school children and caused no unexpected safety issues.
In their analysis, FDA scientists concluded that in almost every scenario the vaccine's benefit for preventing hospitalizations and death from COVID-19 would outweigh any serious potential side effects in children. But agency reviewers stopped short of calling for Pfizer's shot to be authorized.
Most of the study data was collected in the U.S. during August and September, when the delta variant had become the dominant COVID-19 strain.
The FDA review found no new or unexpected side effects. Those that did occur mostly consisted of sore arms, fever or achiness.
However, FDA scientists noted that the study wasn't large enough to detect extremely rare side effects, including myocarditis, a type of heart inflammation that occasionally occurs after the second dose.
According to Rajagopal, parents should be reassured in the vaccine's safety, as doses continue to go through the same protocols required for any other form of vaccine.
"These are tested and safe. It's going through the same steps as the FDA approval and the pending CDC approval. So we really just want to reassure parents, that we're still…the system is still being just as diligent with the COVID vaccine as it has been with any previous vaccine," Rajagopal said.
The agency used statistical modeling to try to predict how many hospitalizations and deaths from COVID-19 the vaccine would prevent versus the number of potential heart side effects it might cause. In four scenarios of the pandemic, the vaccine clearly prevented more hospitalizations than would be expected from the heart side effect. Only when virus cases were extremely low could the vaccine cause more hospitalizations than it would prevent. But overall, regulators concluded that the vaccine's protective benefits “would clearly outweigh" its risks.
While children run a lower risk of severe illness or death than older people, COVID-19 has killed more than 630 Americans 18 and under, according to the CDC. Nearly 6.2 million children have been infected with the coronavirus, more than 1.1 million in the last six weeks as the delta variant surged, the American Academy of Pediatrics says.
Doctors at Advocate Children's Hospital said last week that while cases in children tend to be less severe than those seen in adults, "more children are being hospitalized with severe COVID-19 infection than was seen earlier in the pandemic."
The group also warned that multiple cases of a life-threatening COVID-19-related condition called the pediatric multisystem inflammatory syndrome have been reported in the Chicago area and experts still don't know the long-term effects of COVID-19 on kids.